Laboratory of Drug Informatics

Staff
Kenichi Chonan, Ph.D.Professor
Hirohisa Doi, Ph.D.Associate Professor
Mikie Yamato, Ph.D.Assistant Professor
Risk/benefit communications
Study on risk communication and risk management of drugs
From the viewpoint from the safety of the patients, an evaluation of risk/benefit publichealth improvement, reduction of the economic loss, and the self-medication, it would beimportant in pharmacovigilance that safety issues should be assessed scientifically andshould be communicated effectively and those things would be lead the problem solving As part of a study of that purpose, we conduct an investigation about the riskcommunication of public drug information particularly the safe information for healthcareprofessionals and the patients.
Establishment of evaluation method for Drug information for patients
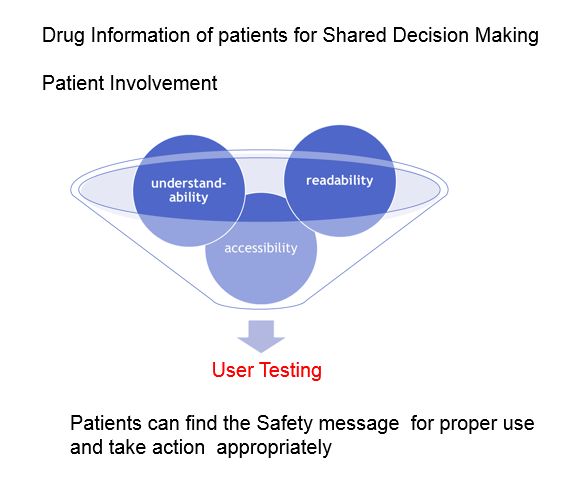
The patients and consumers have desired high quality drug information intheir pharmacotherapy, and are entitled to get it. It is desirable that theinformation should be aimed at shared decision-making between patients andhealthcare professionals about medicines.
The user testing of PILs has been implemented as evidence since 2005so that people can rely on the information provided in the leaflet. Execution ofPILs, which follow the guidance of the user testing, according to the guidanceof the user testing would reflect the views of patients. Good information helpspatients to participate fully in concordant decision-making about prescribed drugs byhealthcare professionals.
In terms of readability, accessibility and understandability, we develop thebest practice of the evaluation method if consumer health and medicalinformation including “Medicine Guides for Patients” would be useful.We should provide information that promotes health in populations andempowers individuals to make sound health decisions.
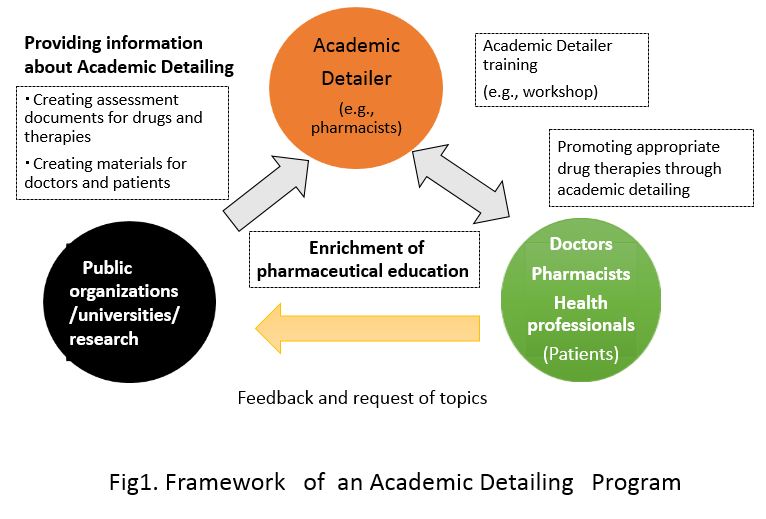
A proper use of drugs –Development of the system for Academic detailing
It is necessary to offer the proper information about prescription drugs forappropriate use of them in clinical practice. However, a lot of time and labor isrequired to comprehensively collect the information necessary for clinicalapplication and it could be extremely difficult. If the clinical experience andother information is derived solely on a commercial basis, then it may lead toimproper prescription practices.“Academic detailing” is a form of interactive educational outreach tophysicians to provide unbiased, non-commercial, evidence-based informationabout medications and other therapeutic decisions, with the goal of improvingpatient care.
In Western countries, the public funds are used to support universities andother research institution programs. The experience from such programsspreads to a broader scientific community.
We make active efforts to the establishment of the information system for the properuse of drugs in the study on model construction of Academic Detailing with ability forinformation literacy up of the individual through interactive communication betweenthe patients, and healthcare professionals.
Predicting Adverse Drug Reaction Utilized Molecular Docking Simulation
One of the main themes of our research is prediction of adverse drug reactionutilized molecular docking simulation.
Molecular docking simulation is one of the most generally tools in drug design. Today’spopular docking programs, such as AutoDock, eHiTS, FlexX, Glide, GOLD, LigandFit,and Surflex, etc. are able to predict protein-ligand complex structure-activity relationship.
By the way, when patients are given medical treatment with drugs, it is sometimesdifficult to continue it, owing to adverse drug reaction.
So, if we can predict adverse drug reaction utilized molecular docking simulation,patients will continue medical treatment with drugs. Specifically, we are now interested inthe structure-activity relationship in 5-HT (5-Hydroxytryptamine) and mACh (muscarinicacetylcholine) receptors as proteins, and drugs as ligands, caused vomiting.